Blood plasma transplants are being used to treat coronavirus patients in the US - here’s how they work

The United States Food and Drug Association (FDA) has now authorised the use of convalescent plasma to treat Covid-19 patients.
The news was announced by the FDA on Sunday 23 August, after Donald Trump criticised health officials for moving too slowly in making the decision last week.
Advertisement
Hide AdAdvertisement
Hide AdHowever, the move is a contentious one, criticised by both FDA health officials and medical professionals.
So how exactly is convalescent plasma used, and is it effective when it comes to fighting coronavirus?
What is convalescent plasma?
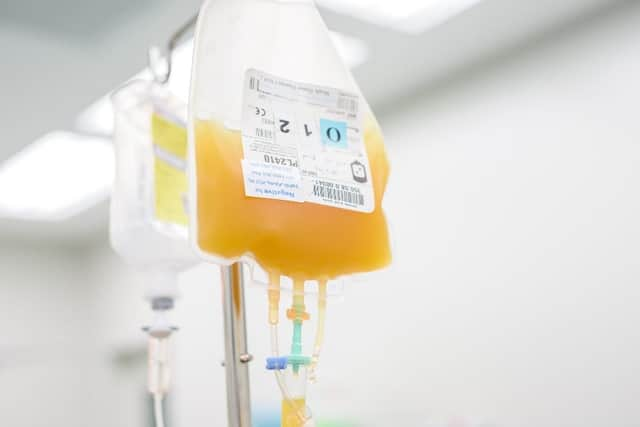
The NHS website describes standard blood plasma as a “yellowish liquid that makes up about half your blood volume.” However, convalescent plasma specifically is slightly different.
Once someone has contracted coronavirus, their blood plasma will contain antibodies which their body will use to help them fight further infection. Convalescent plasma is the name for this antibody-rich plasma.
Advertisement
Hide AdAdvertisement
Hide AdAt the end of March, the FDA set up a convalescent plasma trial, studying its impacts on coronavirus patients. Since then, it has been used to treat over 60,000 Covid-19 patients.
How blood plasma transplants work
Convalescent plasma is taken from the blood of people who have recovered from Covid-19. This antibody-rich plasma is then transfused into patients whose immune systems are not developing enough of their own antibodies in order to fight the virus.
However, like blood donations, stocks of convalescent plasma are currently in very limited supply and require many donors to come forward to replenish them.
What claims have been made about the treatment?
At a White House briefing, Alex Azar (US Health and Human Services Secretary) explained that studies with 70,000 participants had justified the FDA’s Emergency Use Authorization (EUA) of blood plasma transplants.
Advertisement
Hide AdAdvertisement
Hide AdHowever, Azar appeared to be referring to a national study which involved just 35,000 patients who were treated with convalescent plasma.
He said at the briefing, “The data we gathered suggests that patients who were treated early in their disease course, within three days of being diagnosed, with plasma containing high levels of antibodies, benefited the most from treatment.
“We saw about a 35 per cent better survival in the patients who benefited most from the treatment.”
The study appeared on August 12 in a pre-print, rather than a peer-reviewed scholarly or scientific journal. It claimed that about 12 per cent of patients who were treated in the later stages of their disease (four days or more after their diagnosis) had died, whereas only 8.7 per cent of patients who were treated quickly (within three days of diagnosis) had a fatal outcome.
Advertisement
Hide Ad